Services
IROC Core Service Operations
IROC QA centers provide five core services in support of trials with RT or imaging that are part of the NCTN and other NCI‐supported programs.
As trials introduce new technologies or modalities, IROC continually evaluates the core services to best address each trial’s specifications and to develop new improved and efficient QA mechanisms.
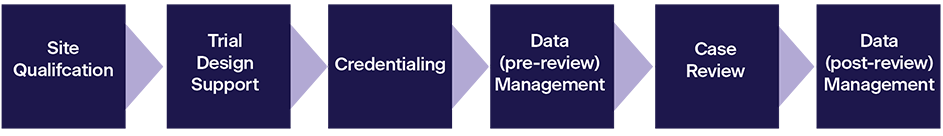
Site Qualification: This service verifies that a site has the basic resources and abilities to participate in NCI supported clinical trials.
Trial Design Support: IROC offers decades of expertise to help NCTN Groups develop new protocols, focusing on sections relating to RT delivery and Imaging, QA, and data collection.
Credentialing: Credentialing is the process of verifying that a specific site and/or clinician/physicist has the knowledge, resources, and capability to meet the protocol specifications.
Data Management (pre‐review): IROC verifies the integrity of submitted data to ensure that the institution has submitted accurate and complete protocol patient data to IROC.
Case Review: Clinical trials require that patients be treated or imaged as specified by the protocol. The purpose of a case review is to verify that this was achieved.
Data Management (post‐review): IROC holds the clinical trial DICOM data for all NCI Groups that use RT for treatment/Imaging. IROC provides comprehensive management and increasingly links the imaging/RT data to the clinical outcome data and trial endpoints.