About Us
We are global leaders in imaging and radiation oncology clinical trial quality assurance.
The National Cancer Institute called for quality assurance core services for imaging and radiotherapy centers to be an integral component of its National Clinical Trials Network. QA leaders and specialists joined forces to establish the Imaging and Radiation Oncology Core (IROC): a single, coordinated program designed to support the NCTN groups in carrying out rigorous, multicenter oncologic clinical trials.
The NCI funds IROC as a member of the National Clinical Trials Network. IROC's mission is to provide integrated radiation oncology and diagnostic imaging quality control programs in support of the National Clinical Trials Network — thereby assuring high-quality data for clinical trials designed to improve clinical outcomes for cancer patients worldwide.
IROC’s charge is to provide:
- Scientific expertise in advanced medical imaging, radiotherapy, and information technology to support establishment of appropriate QA procedures.
- Consultation in the development of research protocols, hypothesis generation and trial design that can be supported by effective QA programs.
- Resources for the efficient collection, qualification, analysis, archiving and transfer of images, radiotherapy plans, and associated clinical data.
- Qualification and credentialing policies and to help ensure the delivery of appropriate protocol-specified radiotherapy and advanced imaging.
In addition to supporting NCTN research activities, IROC may be called upon to carry out quality assurance activities for other NCI-sponsored research.
Infrastructure
IROC brings together the in-depth knowledge, extensive experience, and significant resources of four quality assurance (QA) centers. The resulting full-service QA program provides seamless service delivery to enhance the variety and quality of the QA services we provide. Additionally, IROC benefits from the IT leadership of the American College of Radiology (ACR) and capitalizes on the IT strengths of the individual IROC QA centers.
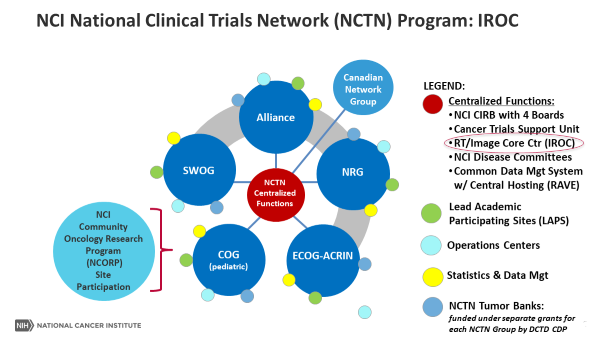
National Clinical Trials Network
The National Clinical Trials Network is composed of four adult and one pediatric cancer cooperative groups:
- SWOG (Southwest Oncology Group)
- The Alliance for Clinical Trials in Oncology (Alliance)
- ECOG-ACRIN (Eastern Cooperative Oncology Group and American College of Radiology Imaging Network) Cancer Research Group
- NRG Oncology (National Surgical Adjuvant Breast and Bowel Project, Radiation Therapy Oncology Group, and Gynecologic Oncology Group)
- Children’s Oncology Group (COG)
The Canadian Cancer Trials Group also partners with these groups.
Leadership
For the overall administration and direction of IROC, two principal investigators (PIs) — Stephen F. Kry, PhD (for radiation therapy) and Michael V. Knopp, MD, PhD, (for imaging) — serve as IROC co-PIs.
Each of IROC's five directors manages the operations of their respective QA centers. The directors are voting members on the IROC Executive Leadership Committee, which is responsible for ensuring the IROC mission is carried out in accordance with the National Cancer Institute's requirements and directions.
- Stephen F. Kry, PhD, University of Texas MD Anderson Cancer Center and Director for IROC Houston, is the IROC Co-PI for RT and the primary contact PI.
- Michael V. Knopp, MD, PhD, University of Cincinnati and Director for IROC Ohio, is the IROC Co-PI for Imaging.
- Thomas J. Fitzgerald, MD, UMass Memorial Health and the University of Massachusetts Chan Medical School, is the Director for IROC Rhode Island.
- Ying Xiao, PhD, Hospital of the University of Pennsylvania, is the Director for IROC Philadelphia (RT).
- Mark A. Rosen, MD, PhD, Hospital of the University of Pennsylvania, is the Director for IROC Philadelphia (imaging).